Essentials of Nursing Children and Young People
Student Resources
Chapter 10: Genertics and epigenetics: effects on children and young people
ANSWERS TO ACTIVITY 10.1: REFLECTIVE PRACTICE
Children’s nurses and health visitors work across a range of settings, at primary, secondary and tertiary levels of care. Think about situations in an acute or community setting when issues to do with genetics have arisen.
Look at Figure 10.1 in the chapter to remind you of the typical areas you may have been on placement. Consider to what extent were you and your mentor able to assess the family's genetics.
Answer: It may be you have been on a placement where the child is being assessed or cared for in relation to one issue, but then genetics issues arise. For example, two children are admitted for surgery. Firstly, Amir, aged 4 years old requires a tonsillectomy. In undertaking his initial assessment pre-operatively, it is noted that he is obese. When you ask about his normal diet, his mother tells you he is always ravenous although she tries to limit his calorie intake. Further questioning reveals he regularly sees a dietician and an endocrinologist who diagnosed Prader Willi syndrome. Looking up Prader Willi syndrome, you find that it is caused by a genetic change. It is typically caused by a deletion of part of the father’s chromosome 15 which is then passed to the child. Undertaking a family pedigree is likely to show that the change is not inherited and is therefore random.
Secondly, Reuben, aged 12, requires dental surgery to remove a number of teeth including one protruding into his hard palate, so his parents and dentist elected for him to have a general anaesthetic. He was also diagnosed with haemophilia shortly after birth. As a result, any injury can mean he bleeds profusely as he lacks a clotting factor. The missing clotting factor can be replaced, but this is easier to do in a hospital.
Undertaking his family pedigree would show that his pattern of inheritance is likely to be traced back through different generations. As an x-linked, autosomal dominant inheritance, it is seen as a condition only in boys although his mother will be a carrier for the condition.
The most famous example of haemophilia inheritance is seen in the royal families of Europe and Russia as a number of Queen Victoria’s children either had or carried the originally mutated gene to their children who then married into the different royal families of Europe and Russia.
Whilst on placement, you and your mentor may not specifically have been the people who assess the child’s pedigree but it is interesting and useful to understand the situation. When you then discuss issues with the family, it can mean it is easier to support them and make appropriate suggestions, e.g. for referral to other services or just in finding out more about the situation they have.
ANSWERS TO SCENARIO 10.1: DWAYNE
Dwayne is a three-year-old who has had intermittent symptoms of feeling tired and lethargic with a racing heartbeat for the last two months. Following investigations, the doctors diagnose a conduction problem in how his heart beats, known as ‘Short Q-T syndrome’. His mum remembers an old discussion that a cousin of hers had a sudden heart attack as a child.
A family pedigree needs to be developed to explore the pattern of inheritance as Dwayne’s condition has a genetic basis. The risk can then be determined for his parents having other children and within the wider family.
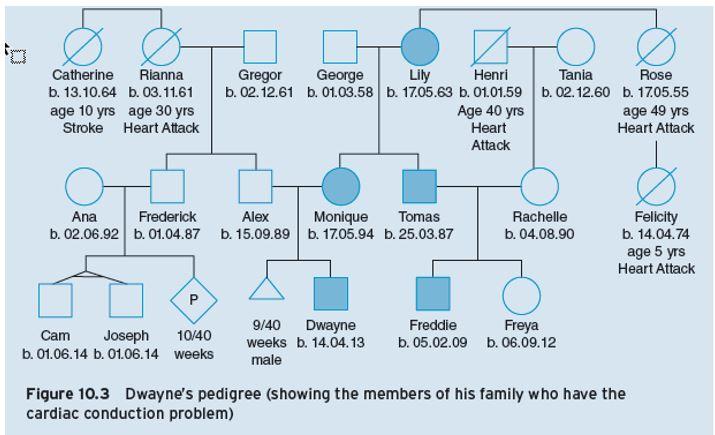
Figure 10.3 – Dwayne’s pedigree (showing the members of his family who have the cardiac conduction problem)
- Identify which members of Dwayne’s family are affected by short Q-T syndrome.
As seen in Dwayne’s pedigree, the condition can be tracked (shaded symbols) through the generations.
- How would you describe the pattern of inheritance seen in Dwayne’s pedigree?
Answers: When you explore the pattern of inheritance you can see that it is seen in both boys and girls, so this single gene defect is unlikely to be X-linked.
In the grandparent generation, George does not have the condition and Lily does. (Note that Lily could be homozygous (RR) for the condition.) Given both their children Monique and Tomas have the condition, the children are likely to both be heterozygous (one copy of the gene, not two).
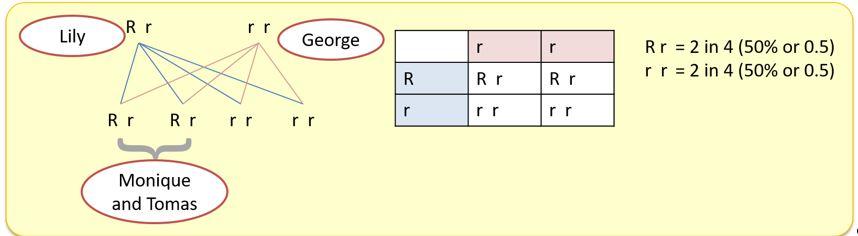
That suggests that this single gene defect is autosomal dominant with the condition occurring wherever the gene is inherited. Tomas’s children (Freddie and Freya) show that Tomas must be heterozygous (one copy of the gene) for the condition.
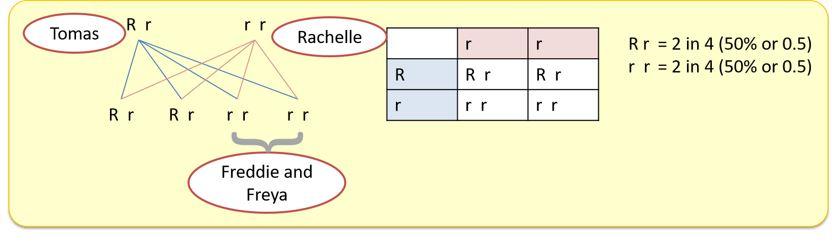
If he was homozygous, then you would expect both children to be carriers of the condition and have the condition. Given neither has the condition, he must be heterozygous and the condition must be autosomal dominant.
ANSWERS TO ACTIVITY 10.2: REFLECTIVE PRACTICE
Using the symbols in Figure 10.2, draw your own family pedigree. Try putting in three generations.
Answer: This is easier once you start to do this, although it does seem daunting. Every person’s family is different, but start with yourself (and partner), go downwards in identifying your children (if any) and then upwards in identifying your parents, your brothers and sisters, then your partner’s brothers and sisters. Finally, try going a generation further back. It does get harder. Try linking in a specific family feature to your tree and see if there are links through the different generations. You could try eye colour or the shape of the nose, even the shape of the thumb. Each of these traits do have genetic components but none are solely related to a single gene change. So, you may find an inheritance pattern, or you may find an anomaly exists in your family because of the role that multiple genes play in inheritance.
The example below represents a possible family where the shaded shapes indicate the people in the family who have a very bent thumb. The pattern seen would suggest that it is not x-linked in that both sexes have the issue and that it is recessive. To have a child, where the two parents are not affected, would either mean that they are both carriers for the gene or the gene has mutated but a mutation is unlikely where these two, uncle and niece, are concerned.
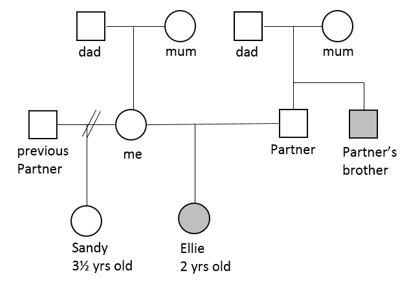
If people have had genetic testing for a specific gene and know they are a carrier for the condition, then a small filled in circle can be placed inside the open circle or square. One example of this would be in conditions such as cystic fibrosis, where parents and siblings may have genetic testing to establish the genetic risk for future offspring they may have.
ANSWERS TO SCENARIO 10.2: DAVID
‘I’m Emma. My child David, who is now 5 years old, was diagnosed with cystic fibrosis following the new-born screening test and then more blood tests in hospital. Although we hadn’t known it, both his dad, Tony and I are carriers for the gene. David has an older brother and sister – Tom aged 12yrs and Katie aged 8yrs – neither has the condition but both are carriers.
Tony has one brother and two sisters. Tony’s dad, David’s grandfather, does not carry the gene but his mother who had died before the genetic testing took place did have a sister with a chronic lung problem who died aged 8yrs old. We think this was probably cystic fibrosis too although it was never confirmed. Of Tony’s mother’s four children, it would appear that one girl is also a carrier for the gene, and other two are unaffected.
On my side of the family, I’m a carrier as is my sister. She now has one child, Suzi, who is unaffected and she’s very reluctant to have any more children in case they become carriers too. Her husband doesn’t have the gene. My parents aren’t alive to ask, but one of them must have been a carrier too.
As a genetic disease, it’s not currently curable but we live in hope that gene therapy will be possible.
- Try drawing David’s family pedigree.
Answer: This is the completed family pedigree for David, how does yours compare to this? The small circles within the larger shape indicate members of David’s family who have been tested for the CFTR gene mutation (7q31.2) and carry the condition.
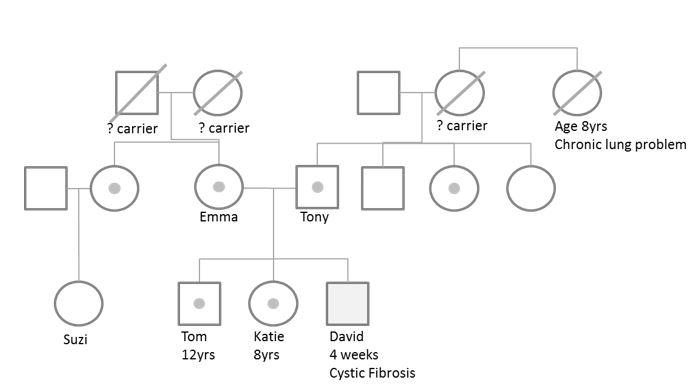
- Who in the family would be a carrier of cystic fibrosis?
Answer: It is unlikely that you will have identified all of the carriers in the family, although you should have identified Emma and Tony as being carriers which would mean that one or both of Emma’s parents are also carriers and also, given that Tony’s dad is not a carrier, that his mother was probably a carrier too. It may be also that Tony’s aunt who had the chronic lung problem and died at 8 years old also had cystic fibrosis, but this information is not known within the scenario so it remains supposition.
- What pattern of inheritance is inherited and why?
Answer: The pattern of inheritance shows that the condition only appears in David and not in preceding generations, making this a recessive inheritance which requires two carriers to produce the condition. It does not appear to be an X-linked issue as Tom does not have the condition. That said, he could have inherited the ‘normal’ genes from his father, not the CF gene mutation. It therefore is likely that this is an autosomal recessive gene pattern of inheritance.
ANSWERS TO WHAT’S THE EVIDENCE? 10.1
In relation to David, the only potential cure is currently through the use of gene therapy. If asked, what should you know about this innovative treatment in order to support the parent or sufferer who asks you?
Gene therapy is the therapeutic delivery of nucleic acid polymers into a patient's cells as a means to treat disease. The polymers are translated into proteins to interfere with, or target gene expression, or to possibly correct genetic mutations.
Gene therapy is a general term to describe a range of techniques designed to tackle the root causes of genetic conditions at source. Methods currently being studied include:
- introducing a working or healthy copy of a gene to replace a mutated gene – this is often done using a virus
- Inactivating or ‘turning off’ a defective gene – as in an autosomal dominant disorder
- introducing a new gene into the body to help combat the disease
The most common form uses DNA that encodes a functional, therapeutic gene to replace a mutated gene. The polymer molecule is packaged within a ‘vector’ which carries the molecule inside cells.
Most gene therapy has focused on treating individuals (somatic cells) but it could be targeted towards sperm or eggs (germ cells). The idea of using germ cells for correction of genetic problems has caused ethical concerns.
- Is it appropriate to change the human genome for future generations?
Answer: Please visit the source below to find out more about this:
Ledford, H. (2016) Gene Editing is Just the Beginning is an excellent introduction to the future of gene therapy. Available at: www.nature.com/news/crispr-gene-editing-is-just-the-beginning-1.19510
ANSWERS TO ACTIVITY 10.3: CRITICAL THINKING
On a recent insight visit, you encountered the following situations where the health professionals need to consider the balance between the conflicts that inevitably arise in practice. Based on the issues you have just read about, what are the ethical, legal and societal questions that arise for each case?
- Prenatal diagnosis
Sunil and Lucy are expecting a second baby and have requested sexing of the foetus with a view to termination if it’s a boy. Their first child has a condition they know is more common in boys. Overall, the risk of having another child with the same condition is 5 per cent (1 in 20)
Answer: Sunil and Lucy’s request would cause a dilemma. Justice is a concept that emphasises fairness and equality. Patients in similar situations should be treated similarly, and in a broader sense, benefits and costs should be fairly distributed across a population. The risk for this child, and for Sunil and Lucy’s future children is low – at 1 in 20 – but may not feel so the couple, particularly if they are struggling to help their son. In any case, there is no guarantee that a daughter would not have the condition even if it is more prevalent in boys.
- Children and predictive testing
Daniel and Isha have requested testing to see if their child has inherited an adult onset autosomal dominant condition that they have just found out runs in the family.
Answer: Health staff should consider the principle of beneficence, or acting in the child’s best interests. As a general rule the benefit should outweigh the harm and testing is best left until the child is old enough to consent. Testing children less than 12 years of age is not generally justified unless there is a clear benefit in terms of immediate medical intervention, or the need to begin a form of therapy early to prevent secondary problems.
The principle of non-maleficence – the principle of not doing harm – or leaving Daniel and Isha worse off than before a course of action should be considered throughout.
- Implications for individuals, immediate and extended family
Sam is a 14-year-old boy who comes to clinic with his father after a referral from Sam’s GP. Sam’s Dad wants him to have genetic testing for Type 1 osteogenesis imperfecta but Sam is reluctant. Sam has an older sister who is due to get married and wants to start a family before her career takes her overseas.
Answer: Sam would need to sign consent. This needs to be obtained before actions such as clinical photography, genetic testing and storage of DNA. Although Sam’s parent can sign as they have parental responsibility and he is under 16, in practice, if Sam is deemed to have the capacity to consent his wishes would be paramount.
The Human Tissues Act 2004 says that consent should be formally obtained where cellular material is used to obtain genetic information for another person. In the clinical setting children and teenagers are often the candidates for testing. Some will have learning difficulties and may lack capacity to give consent.
The Mental Capacity Act (2005) England and Wales came into force in England and Wales in 2007. It replaced the previous case law and there is a legal duty to use the legislation and apply the ‘Test for Capacity’ for any relevant decision for people who lack capacity. Decisions should reflect the best interest of the patient but also take into account the interests of the wider family. In England and Wales, the Court of Protection can appoint an appropriate person to act on someone’s behalf, but in Scotland it is legal for certain designated adults, including family members, to consent or refuse on behalf of a person lacking capacity.
If Sam can understand the relevant information and can use this to inform his decision, then his wishes should be respected.
Sam should be empowered when decisions need to be made. This means he needs accessible information but should feel free to opt out of genetic testing at any stage.
Privacy/truth telling/duty of care/right to know versus right not to know – there are many issues relating to a genetic disease that a family may wish to keep private or to share. Sam’s sister is reported to be planning a family. There is the difficulty in sharing results between regional genetics services when many family members are seen. Confidentiality would only be breached under extreme circumstances.
Families should be given full information including risks, limitations, outcomes, and implications of genetic testing and the option of not participating. The consequences of each course of action should be explored.
References
Human Tissues Act 2004 (c.30) Great Britain. London: HMSO
Mental Capacity Act 2005 (c.9) Great Britain. London: HMSO
